A further increase of the fuel temperature to values close to the boiling point may result in thermal decomposition, ie pyrolysis, leading to the formation of coke-like residues, named cenospheres (see section "4.4.3. Coke"). Because of these carbonaceous bodies formed during combustion, radiation plays a more important role than in light fuels, where such effects can be neglected.
As a result, the droplet size during the combustion process is not governed by a D2 law as in volatile fuels (see section "2.3.2. Free droplet technique"):
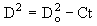
where the droplet diameter at time of combustion t can be calculated from the initial droplet diameter Do and a proportionality constant C.
Four major inconveniences arise when firing heavy fuels:
In the case of emulsions the physical effects of water addition lead to better combustion properties by improved atomisation (Jordan and Williams (1981), Marcano (1992)) . Micro-explosions are produced as the result of the formation, growth and bursting of vapour bubbles within the superheated droplet. Since the oil can sustain very high temperatures during combustion, the water droplets can be superheated. The emulsion droplet is eventually shattered by the internal formation of water bubbles and their rapid vaporisation. This process is called secondary atomisation, which increases the evaporation surface area and the mixing of the burning species in air. The amounts of particulates and smoke formed are minimised.
 Previous |  Table of Contents |  Next |